With Lilly Korea’s Lartruvo (ingredient: olaratumab) obtaining insurance benefits, the combination of Latruvo and doxorubicin might become a standard first-line treatment for soft tissue sarcoma, an oncology professor said.
Lartruvo received approval from the Ministry of Food and Drug Safety in March last year. Latruvo sells at 1,064,000 won ($978.8) per vial, with its insurance taking effect from this month.
The drug can be used in patients with locally advanced or metastatic soft tissue sarcoma (excluding gastrointestinal stromal tumors, Kaposi sarcoma, and osteosarcoma) who have not used anthracycline-active anticancer therapy before. The insurance coverage is available together with doxorubicin only.
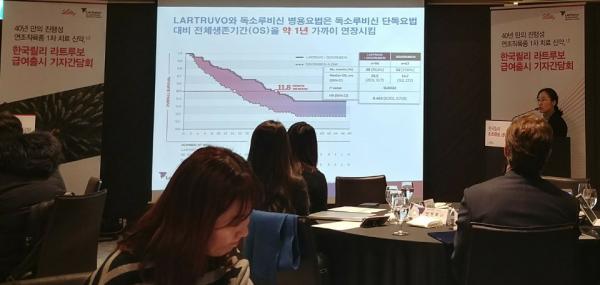
“Prolonging the overall survival by one year (compared with the existing standard therapy) was unprecedented in sarcoma studies,” said Ahn Jin-hee, a professor of oncology at Asan Medical Center, at a news conference by Lilly Korea at Sheraton Seoul Palace Gangnam Hotel, Friday. “With the insurance coverage, the existing first-line treatment for advanced soft tissue sarcoma involving anthracycline-based chemotherapy will be changed to the combination of Lartruvo and doxorubicin.”
The Lartruvo and doxorubicin combo raised the overall survival by 11.8 months to 26.5 months, compared with doxorubicin-alone therapy, in clinical trials. The median progression-free survival was 6.6 months, 2.5 months longer than that of the control group.
“The combination therapy did not show any major side effect, compared to monotherapy. Even if we consider international guidelines (NCCN, National Comprehensive Cancer Network), it is highly likely that the Lartruvo and doxorubicin combo will be chosen as the first-line therapy. Most patients who can use doxorubicin will receive the combo treatment," Ahn said.
She went on to say that priority in treating soft tissue sarcoma is extending the overall survival.
“Lartruvo is the first drug in 40 years to extend the survival by nearly one year, with the first-ever monoclonal antibody with a different mechanism called PDGFR-α antibody," Ahn added.
Lartruvo received accelerated approval from the U.S. Food and Drug Administration and European Medicines Agency to be used together with doxorubicin in 2016.

