First company deprived of insurance benefits under ‘two strike-out’ rule
The Ministry of Health and Welfare has decided to impose the fine of 55.1 billion won ($48.6 million) on Novartis Korea and suspend insurance benefits for nine of its products, for dishing out illegal rebates.
The move followed a district prosecutor office’s indictment of Novartis last August, which accused the company of providing 2.59 billion won in illegal rebates to doctors over the previous five years, the ministry said Thursday. The company faced the similar charges in 2011 for providing 7.2 billion won in kickbacks.
Novartis Korea has become the first company operating in the nation to be dealt with the heavy penalty since the government introduced the “two strike out system” in July 2014, which called for harsh punishment of companies that bribe officials.
In disciplining the company, the ministry saw that the burden of penalty falls mainly on the company, not on its patients, it said. As the first company to have violated the stricter rule, Novartis is also the first company to have nine drugs delisted from insurance payment, under Article 41-2 of the National Health Insurance Act.
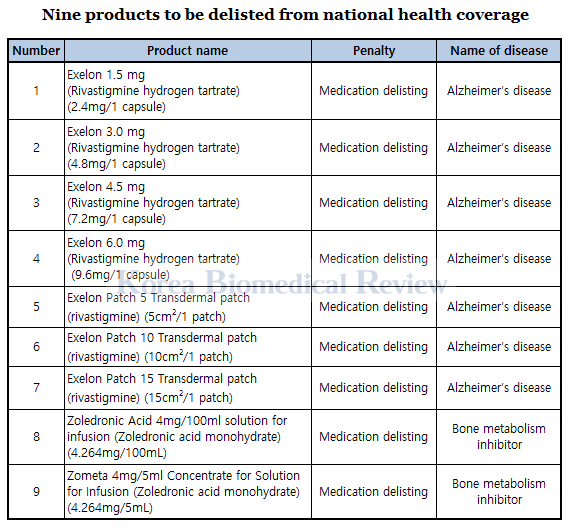
According to the ministry, its decision to punish the company mainly with fines instead of delisting its drugs has reflected the opinions of related organizations, clinical trial professionals, and patient groups. Particularly the patients who were forced to switch to less effective generics have vehemently protested against Novartis Korea for its irresponsible and unethical behavior, calling for the ministry to lay a heavy hand on the latter.
Aside from nine products that have been delisted from insurance coverage for six months as they have substitutes, the ministry has kept 33 out of Novartis’ total 42 products insured because they have no alternative drugs.
The ministry plans to give a maximum three-month grace period to prevent problems in patient treatment, considering the time required for additional production, distribution, bidding, and purchasing of substitutes by other companies and distributors,
Regarding the delisted drugs, the ministry said it had ensured output and distribution channels of substitutions for patients in need.
“We will respond firmly to illegal rebates by holding both recipients and providers accountable, and improve the effectiveness of sanctions by strengthening cooperation with related institutions,” a ministry official said. “We are also looking to increase the ceiling of penalties from 40 to 60 percent and the scope of drug price reduction from 20 to 40 percent.”
The ministry plans to discuss how to improve the rebate sanction and penalty system with the National Assembly to find more efficient ways and stop the spread of illegal kickbacks.

