The Korea Centers for Disease Control and Prevention (KCDC) has approved two additional detection kits developed by domestic diagnostics companies, SolGent and SD Biosensor, for urgent diagnosis of the novel coronavirus.
The KCDC said Thursday that it gave the go-ahead to SolGent's DiaPlexQ Novel Coronavirus (2019-nCoV) Detection Kit (DiaPlexQ) and SD Biosensor STANDARD M n-CoV Real-Time Detection Kit for emergency COVID-19 test.
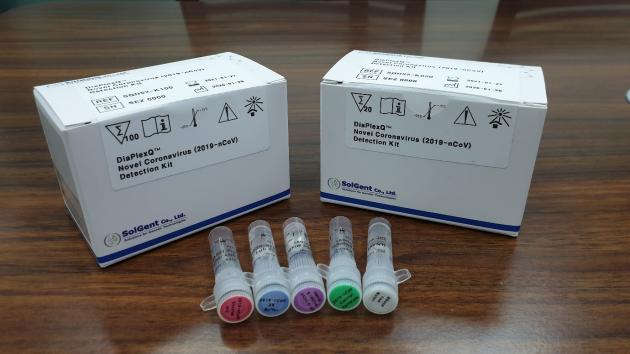
SolGent's DiaPlexQ is a medical device for in vitro qualitative detection of the new coronavirus gene (ORF1a gene and gene) from the samples of suspected patients. The device can diagnose quickly within two hours after extracting RiboNucleic Acid (RNA).
SD Biosensor's STANDARD M n-CoV Real-Time Detection Kit takes about six hours to finish the qualitative detection of the COVID-19 gene (E gene and RNA-dependent RNA polymerase gene) from the suspected patients' specimens.
Korea now has four types of detections kits available for pressing diagnosis of COVID-19.
On Feb. 4, the KCDC approved Kogene Biotech's Powerchek 2019-nCoV Real-time PCR kit and Seegene's Allplex 2019-nCoV Assay for diagnosing COVID-19. These devices also detect the E gene and the RNA-dependent RNA polymerase gene.
Emergency use approval is a system that temporarily authorizes the use of reagents developed to a certain level when the nation faces the threat of pandemic without a domestically approved detection kit.

