Boryeong Pharmaceutical said Wednesday that it would release the secondary hyperparathyroidism treatment, Pacitol (Ingredient: paricalcitol), on April 1.
Pacitol injection is the first generic treatment to deal with secondary hyperparathyroidism related to chronic renal failure. The company won the license for making and selling the product from the Ministry of Food and Drug Safety on Jan. 30. The dosage was released as 5 micrograms per milliliter, and the company produces the treatment itself.
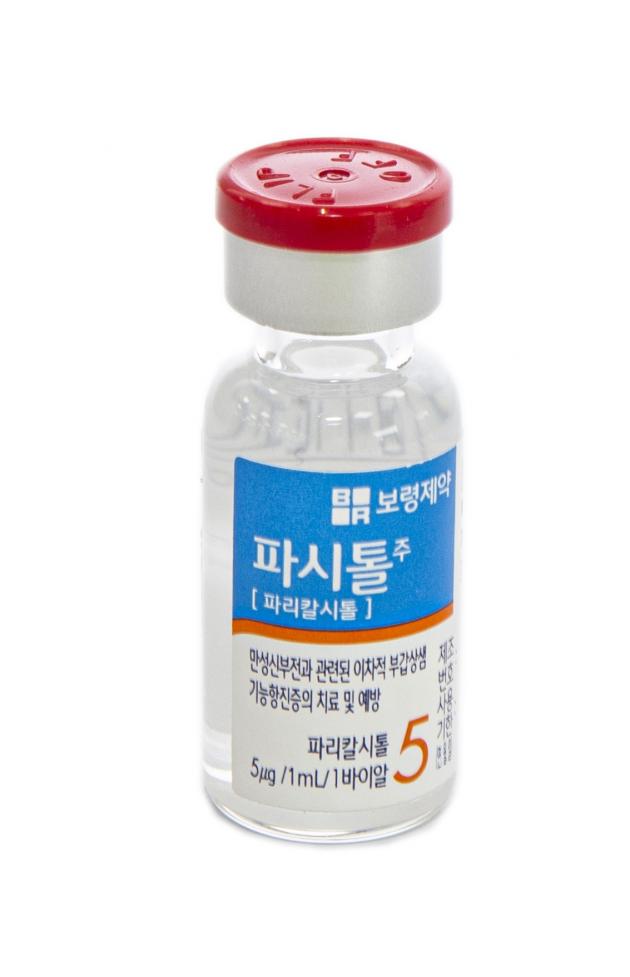
Pacitol injection will be launched in a vial form for the users' convenience and safety. The existing paricalcitol component treatment has been released in a glass ampoule and caused concerns about small glass fragments generated and mixing with the treatment when opened.
Hyperparathyroidism, which occurs mainly in dialysis patients due to chronic renal failure, is a condition in which parathyroid hormone is secreted excessively to control hypocalcemia and hyperphosphatemia.
That, in turn, can result in imbalance of calcium and phosphorus levels in the body and cause severe complications.
"The range of drug choices in the medical field has expanded with the release of the first generic of the paricalcitol," said the company’s product manager for pacitol. "We expect vial-type pacitol's safety and low price will help both the medical providers and patients.

