Local researchers said they have successfully developed a new eco-friendly and low-cost method of synthesizing chemical drugs.
The research team, led by Professor Park Cheol-min of the Department of Chemistry at Ulsan National Institute of Science & Technology (UNIST), said Thursday it developed a synthesis method to make "indolopyran," one of the nitrogen ring compounds, using electric energy.
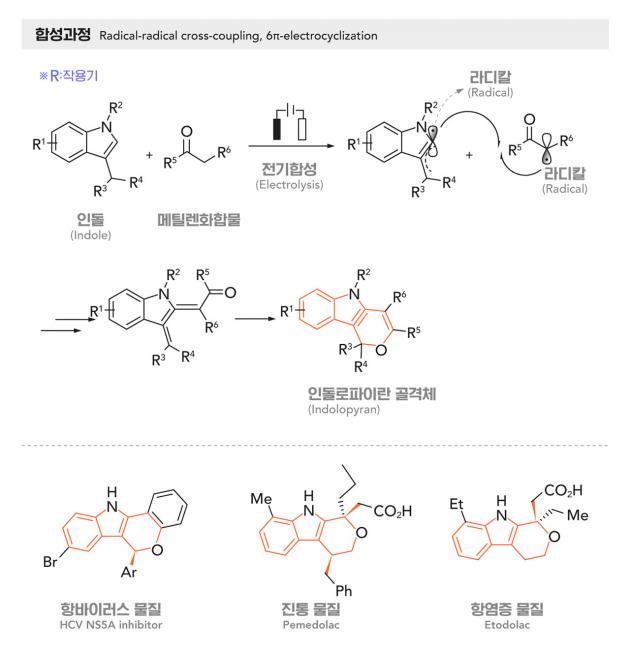
Nitrogen ring compounds account for 60 percent of the medicines recently authorized by the U.S. Food and Drug Administration. Indolopyran, in particular, has drawn attention with its anti-cancer and anti-inflammation effects, but they are difficult to synthesize. Synthesis for indolopyran requires a base and a metal catalyst, and it occurs only at high temperatures.
The research team has excluded factors such as metal catalysts and high temperatures that cause environmental pollution. Instead, they developed a green and economical method using oxygen to induce reaction.
The researchers used the electrode’s anode and cathode reaction to synthesize indoles with active methylene compounds. For this, they used oxygen as a reagent at room temperature and iodide as a catalyst.
This synthesis method has an advantage that oxygen in the air can be used as a pre-base. Also, the constant voltage mode solved the problem of functional group damage caused by high voltage.
Choi Su-bin, a researcher at the UNIST’s Chemistry Department, said instead of the conventional synthesis method, which mainly uses only one pole, the team used both the anode and the cathode's reactions synthesize structurally complex indolopyran.
Based on the discovery, the team applied the same method to synthesize “dihydrofurans.” Dihydrofuran is a frame to which a functional group binds to a drug.
Instead of indoles, the research team used enamines to synthesize dihydrofurans that included oxygen-containing pentagonal carbon ring.
Professor Park said his team could use an eco-friendly and economical way to synthesize nitrogen ring compounds with electric energy, compared to conventional synthesis methods.
“It is also meaningful that we suggested a method for synthesizing various ring-shaped structures from various starting materials by finding the reaction routes,” he said.
The paper, titled, “Electrosynthesis of Dihydropyrano[4,3-b]indoles based on Double Oxidative [3+3] Cycloaddition,” was picked as the Hot Paper of Angewandte Chemie International Edition and published on July 7.
The National Research Foundation of Korea supported the study.

