Novavax unveiled the successful results of the phase-1 clinical trial on a Covid-19 vaccine candidate, NVX-CoV2373, Wednesday. The company said its vaccine candidate showed immunogenicity safely on day 35.
The outcome is expected to give an advantage to SK Bioscience, which earlier clinched a deal with the U.S. biotech firm to manufacture the experimental vaccine and supply it globally.
The New England Journal of Medicine (NEJM) on Wednesday published the article, titled, “Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine.”
NVX-CoV2373 is a nanoparticle vaccine candidate made by expressing the COVID-19 spike protein in insect cells with recombinant technology. Administering an inactivated vaccine to the human body can induce neutralizing antibodies.
Novavax said it used its immunity booster, Matrix-M, as adjuvant therapy in the trial to enhance the effect of generating neutralizing antibodies.
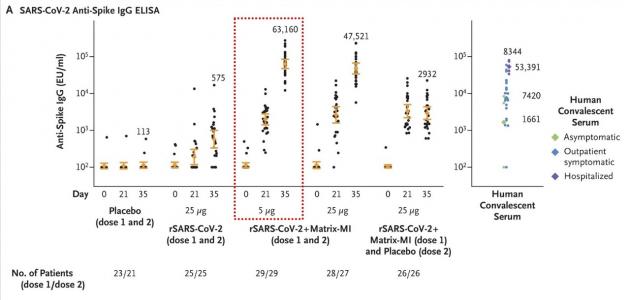
The study evaluated the vaccine candidate’s efficacy and safety in 5-μg and 25-μg doses in 131 healthy adults. After randomization, 83 participants received the vaccine with adjuvant and 25 without adjuvant, and 23 received the vaccine with placebo. The participants had two injections of the investigational vaccine, 21 days apart.
According to the results, the participants did not have any serious adverse events. Reactogenicity was absent or mild in the majority of participants, more common in those with adjuvant Matrix-M for a short duration, less than two days on average. The addition of Matrix-M enhanced immune responses, allowed the dose sparing of the vaccine, and induced T helper 1 (Th1) response, the NEJM article said.
“At 35 days, NVX-CoV2373 appeared to be safe, and it elicited immune responses that exceeded levels in Covid-19 convalescent serum,” the researchers concluded. “The Matrix-M1 adjuvant-induced CD4+ T-cell responses that were biased toward a Th1 phenotype,” they added.
Based on the latest data, Novavax has begun a phase-2 trial and is reportedly preparing for a phase-3 study. The company aims to enter the phase-3 trial in October.

